
Electronegative ligands such as F will always go to the axial sites. A molecule, that is sp3d hybridized and has a molecular geometry of linear, has bonding groups and lone pairs around its central atom. Seesaw Molecular Geometry - The seesaw shape maximises the bond angles of the single lone pair and the other atoms in the molecule. Study with Quizlet and memorize flashcards containing terms like The Lewis dot symbol consists of the symbol for the element surrounded by dot(s). Disphenoidal or seesaw (also known as sawhorse 1) is a type of molecular geometry where there are four bonds to a central atom with overall C 2v molecular symmetry. Transcribed image text: A molecule with a seesaw molecular geometry has a bond angle of A) <120 degree for equatorial bonds and <90 degree for axial bonds. In general, by this reasoning, lone pairs and electropositive ligands such as CH 3 will always prefer the equatorial sites in the trigonal bipyramidal geometry. Bond angle (s) Ideal ax-ax 180, eq-eq 120, ax-eq 90. Give the hybridization for the Br in BrCl3.
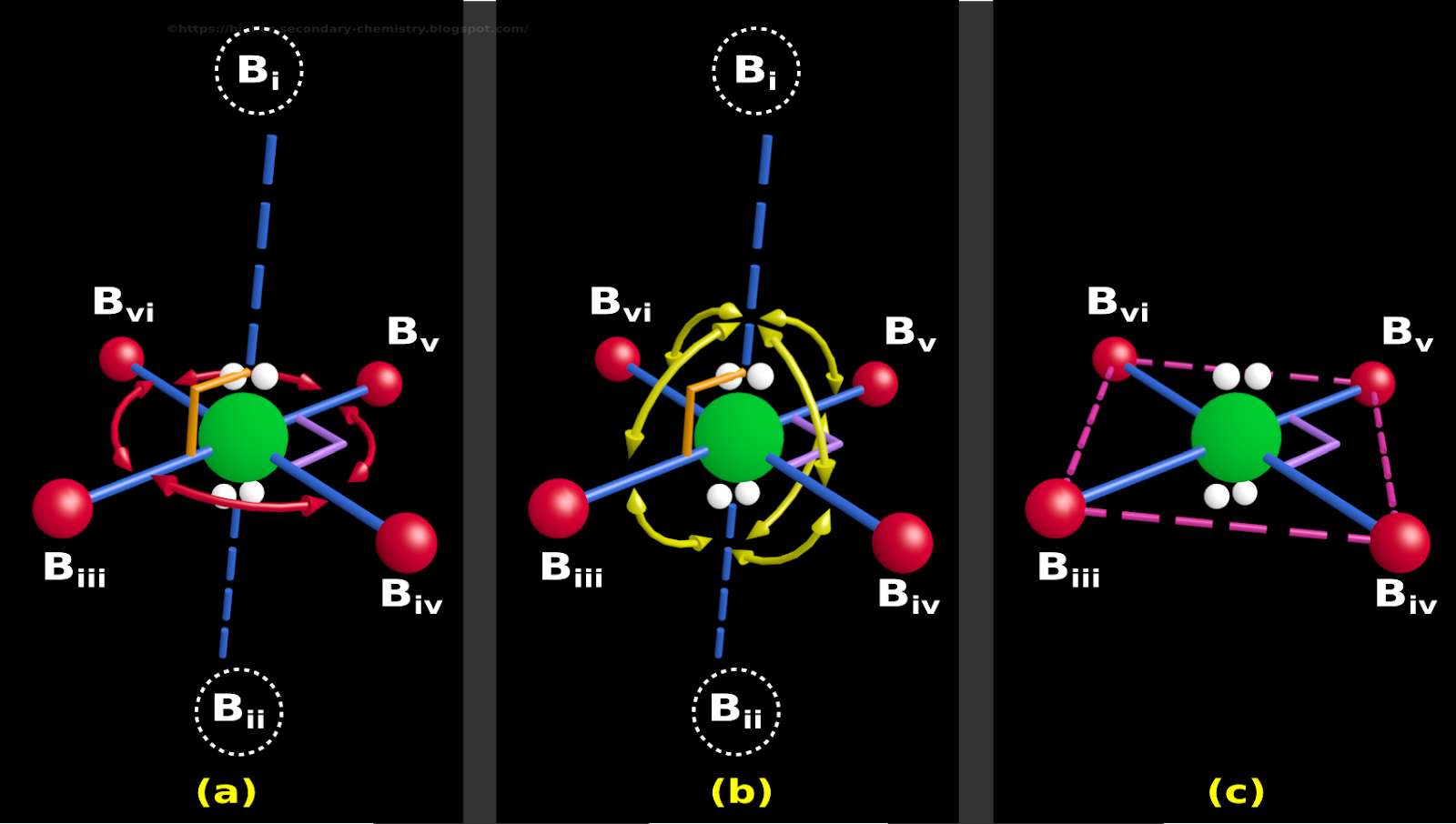
Which of the following is the best statement about the bond angle indicated by the double arrow in the diagram.

= 0.867 \:bond (formal \: charge = -0.122)\)īecause fluorine is more electronegative than a lone pair, it prefers the axial site where it will have more negative formal charge. A molecule, that is sp3d2 hybridized and has a molecular geometry of square pyramidal, has bonding groups and lone pairs around its central atom. Transcribed Image Text: Below is a cartoon drawing of a seesaw molecular geometry which is based upon the trigonal bipyramidal electron group geometry.


 0 kommentar(er)
0 kommentar(er)
